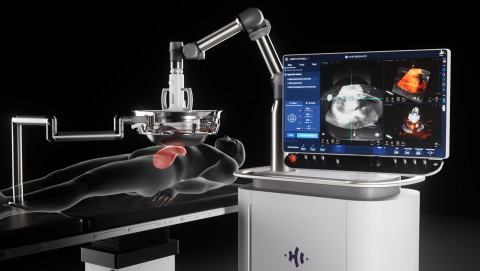
 |
| HistoSonics Edison Histotripsy System (Photo: Business Wire) |
HistoSonics, (www.histosonics.com), the manufacturer of the non-invasive Edison? Histotripsy System, announced today a patient from the University of Rochester Medical Center has received the world’s first targeted liver tumor treatments utilizing the Edison System following its recent FDA De Novo clearance. In the same week Cleveland Clinic treated their initial patients suffering from liver tumors utilizing histotripsy. HistoSonics' image guided sonic beam therapy system uses proprietary technology and advanced imaging to deliver non-invasive, personalized treatments with precision and control. The science of histotripsy uses focused sound energy to produce controlled acoustic cavitation that mechanically destroys and liquifies targeted tissue at sub-cellular levels.
On December 18th, Roberto Hernandez-Alejandro MD, Chief of the Division of Transplantation, Koji Tomiyama, MD, PhD Associate Professor of Surgery & Transplant, and team were first to use Edison to perform a histotripsy liver treatment on a patient suffering from recurrent liver tumors originating in the colon. “There is an enormous potential applicability of histotripsy in patients with liver tumors. By destroying targeted liver tumors, histotripsy opens the opportunities for patients to be downstaged and bridged for surgical resections and transplantation,” said Dr. Hernandez-Alejandro. He added, “One of the most interesting findings in our early experience is the preservation of the vessels and blood flow, which is unique and may open a new era in liver therapies.”
C.H. David Kwon, MD, PhD, Director of Minimally Invasive Liver Surgery at Cleveland Clinic, and his team performed their initial procedure on a patient with primary colorectal disease with metastasis to the liver that has continued to progress despite getting chemotherapy. “In our early experience, we have found that patients have the potential to benefit from histotripsy because it is less invasive than other treatment options and our patients reported a lack of pain following the procedure,” said Dr. Kwon. The Cleveland Clinic team led by Dr. Kwon, Federico Aucejo, MD, and Jaekeun Kim MD has treated three patients with liver tumors of multiple different diseases.
“Our purpose has always been to make a meaningful change in the lives of patients,” said Joe Herman MD, Medical Director. Herman added, “Working with the University of Rochester Medical Center and Cleveland Clinic as the first to offer non-invasive histotripsy to treat their patients’ liver tumors as part of standard clinical practice supports our ongoing mission.” HistoSonics is in the process of training key staff and launching over a dozen of its first partners programs around the United States. The company believes that the novel mechanism of action of their proprietary technology provides significant advantages to patients, including the ability of the treatment site to recover and resorb quickly. Uniquely, the HistoSonics’ platform also provides physicians the ability to monitor the destruction of tissue under continuous real-time visualization and control, unlike any modality that exists today.
For more information on the University of Rochester Medical Center Histotripsy and Liver Surgery Team: University of Rochester Medical Center Liver Surgery and Transplant (https://www.urmc.rochester.edu/cancer-institute/cancers/liver.aspx)
For more information on the Cleveland Clinic Liver Surgery Team: Cleveland Clinic Liver Surgery and Transplant (https://my.clevelandclinic.org/departments/transplant/programs/liver)
The Edison? System is intended for the non-invasive mechanical destruction of liver tumors, including the partial or complete destruction of unresectable liver tumors via histotripsy. The FDA has not evaluated the Edison System for the treatment of any specific disease or condition. Use of Edison System in kidney applications is limited by federal law to investigational use.
리서치뉴스 임원호 기자 ipi@uelt.net
|